A Novel Epigenetic Function of WEE1
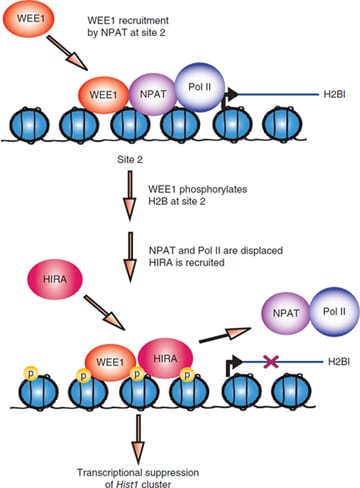
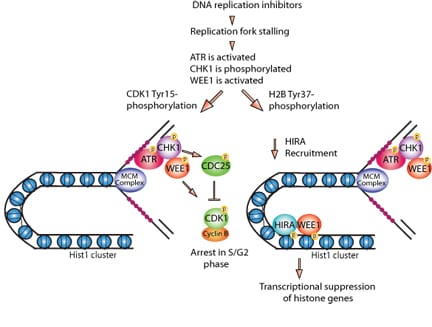
We discovered that WEE1 kinase phosphorylates histone H2B at Tyr37 in a short window of 30-40 minutes in late S phase. All eukaryotic cells precisely regulates histone levels by shutting off histone transcription at the end of DNA synthesis. We obsevered that WEE1 kinase deposit pY37-H2B epigenetic marks upstream of Hist1 cluster, suppressing global histone transcription in both yeast and mammalian cells.
Our data provides crucial evidence that in addition to mRNA turnover, histone transcription shut off would efficiently lower histone transcript levels once DNA synthesis is complete, thus eliminating overproduction of core histones. Thus, WEE1, a cell cycle regulator has a dual role as an epigenetic modifier that maintains histone transcript levels.
- Mahajan K, Fang B, Koomen JM and Mahajan NP*. H2B Tyr37 phosphorylation suppresses expression of replication-dependent core histone genes. Nature Structural & Molecular Biology 2012
WEE1 epigenetically regulates 5hmC levels
WEE1 kinase has been reported to be aberrantly expressed in melanomas, glioblastoma multiforme (GBMs), triple negative breast cancers (TNBCs) and prostate cancer. Dependence of various cancer cells on WEE1 signaling suggests that targeting epigenetic activity of WEE1 is a viable strategy.
Clinical significance of WEE1-H2B epigenetic signaling in Melanoma, GBM and prostate cancer
WEE1-H2B epigenetic signaling plays a crucial role in various malignancies such as melanoma, GBM and prostate cancer